Generic Drugs vs. Brand-Name Medications: What You Really Need to Know
When you pick up a prescription, you might see two pills that look completely different-one with a big name on it, another with a plain label and a much smaller price tag. You’re probably wondering: generic drugs really work the same as brand-name ones? Or is this just a cost-cutting trick?
The short answer? For most people, yes. Generic drugs work the same. But there are important exceptions, and knowing them could save you money-or even prevent a health problem.
What Exactly Is a Generic Drug?
A generic drug is the exact chemical copy of a brand-name medication. It has the same active ingredient, same strength, same way it’s taken (pill, injection, cream, etc.), and it works the same way in your body. The FDA requires this. It’s not a copycat. It’s a legal, regulated, and tested duplicate.
Think of it like this: if brand-name ibuprofen is a Ford Mustang, the generic version is a Toyota Camry. Different looks, same engine. Both get you from point A to point B. The difference? The Mustang costs $35,000. The Camry costs $22,000. And in the drug world, that gap is even bigger.
Generic drugs became common after the 1984 Hatch-Waxman Act in the U.S. Before that, companies could lock up a drug’s formula with patents forever. The law changed that. It let other companies make the same drug once the patent expired-so long as they proved it worked just as well. No need to run new clinical trials on thousands of people. Just prove the body absorbs it the same way. That’s called bioequivalence.
How Much Do Generics Actually Save You?
On average, generic drugs cost 80% to 85% less than their brand-name versions. That’s not a guess. It’s backed by data from the FDA and GoodRx. For example, the brand-name cholesterol drug Lipitor used to cost around $130 a month. After generics hit the market, the price dropped to under $1 a month. That’s not a sale. That’s a revolution.
From 2007 to 2016, generic drugs saved the U.S. healthcare system $1.67 trillion. Medicare alone saved $77 billion. Medicaid saved nearly $38 billion. These aren’t small numbers. They’re life-changing for people who have to choose between paying for medicine or paying rent.
Today, about 9 out of every 10 prescriptions filled in the U.S. are for generic drugs. That’s not because doctors are pushing them. It’s because patients and pharmacies choose them. And for good reason.
Are Generics Just as Safe and Effective?
The FDA holds generic manufacturers to the same standards as brand-name ones. Same quality checks. Same clean factory rules. Same testing for strength, purity, and stability. In fact, many brand-name companies make their own generic versions after the patent expires.
A 2016 study in JAMA reviewed over 2,000 bioequivalence tests. The average difference in how the body absorbed the drug was just 3.5%. The FDA allows up to 25% variation. So generics don’t just meet the bar-they hit it comfortably.
For common drugs like metformin (for diabetes), lisinopril (for blood pressure), or atorvastatin (for cholesterol), patient reviews show 87% report no difference between generic and brand. That’s a solid track record.

Where Generics Can Be Tricky
But here’s the catch: not all drugs are created equal.
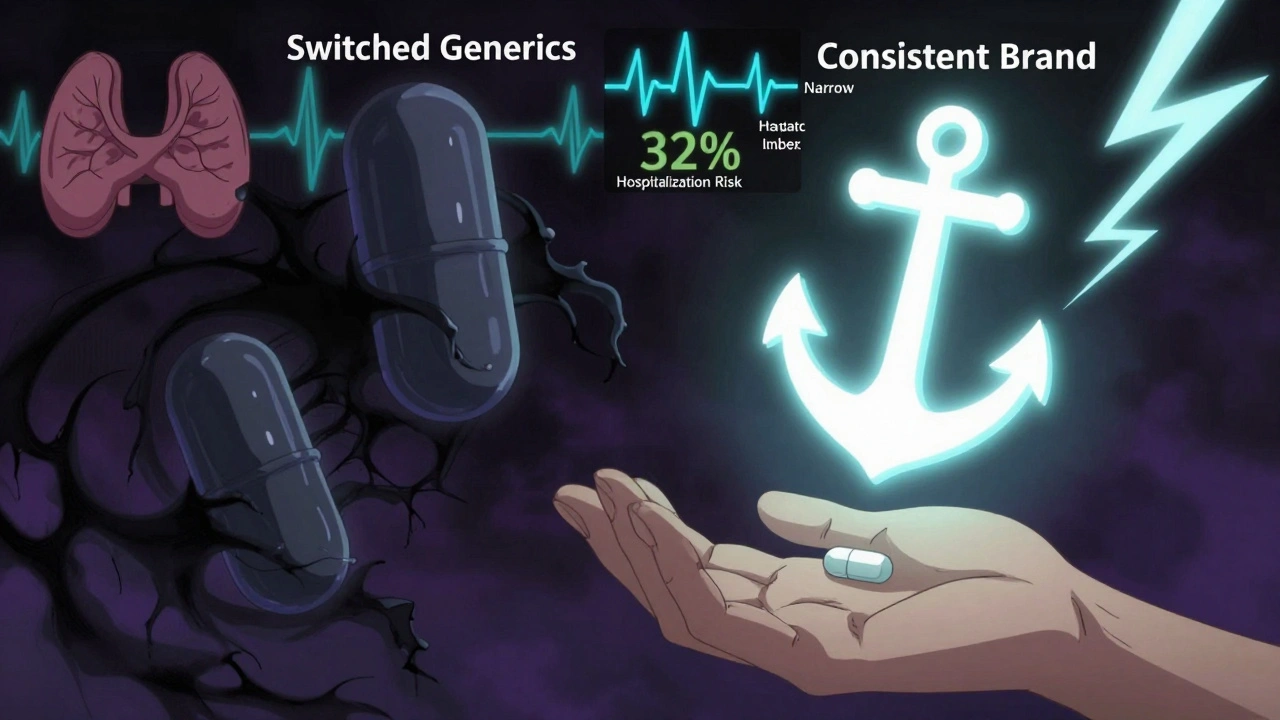
Some medications have what’s called a narrow therapeutic index (NTI). That means the difference between a dose that works and one that’s dangerous is tiny. Even a small change in how your body absorbs the drug can cause problems.
Examples include:
- Warfarin (blood thinner)
- Levothyroxine (for thyroid)
- Phenytoin and levetiracetam (for seizures)
For these, switching between different generic brands-even ones approved by the FDA-has been linked to problems. One study found patients on levetiracetam had a 32% higher risk of being hospitalized for seizures after switching between generic versions.
Why? Because while the active ingredient is the same, the fillers, dyes, or coating might be different. For most people, that doesn’t matter. But for someone with a sensitive system-like a person with epilepsy or thyroid disease-it can.
That’s why some neurologists and endocrinologists recommend sticking with one brand of generic-or even the original brand-if it’s working well. It’s not about quality. It’s about consistency.
Why Do Generics Look Different?
Ever opened a bottle and thought, “This isn’t the same pill!”? That’s normal.
By law, generic manufacturers can’t make their pills look exactly like the brand-name version. That’s to avoid trademark infringement. So your generic metformin might be blue and oval, while the brand is white and round. Same drug. Different shape. Different color. Different markings.
But here’s the problem: 65% of medication errors involving generics happen because patients think the different-looking pill is something else. They stop taking it. Or they think it’s a mistake and don’t refill.
Always check the label. Know the name of the active ingredient. Keep a list of your meds, including the shape and color, so you notice changes. If you’re unsure, ask your pharmacist.
What About Biosimilars?
There’s another category you might hear about: biosimilars. These are the generic versions of biologic drugs-complex medicines made from living cells, like Humira (for arthritis) or Enbrel.
Unlike simple pills, biologics can’t be copied exactly. So biosimilars are “highly similar,” not identical. They’re still cheaper-often 15% to 35% less than the brand. But they’re not as widespread. Only about 3% of biologic prescriptions are filled with biosimilars, even though 35 have been approved by the FDA.
Why? Cost. Insurance companies don’t always push them. Doctors aren’t always trained on them. And patients are nervous. But they’re safe. And they’re growing fast. Humira’s patent expired in 2023, and its biosimilars are already cutting costs across the U.S.
What You Can Do
Here’s how to make smart choices:
- Ask for the generic. Unless your doctor says otherwise, always ask if a generic is available. It’s your right.
- Check the active ingredient. Make sure the generic has the same one as the brand. You can find this on the bottle or by asking your pharmacist.
- Stick with one manufacturer. If you’re on a narrow therapeutic index drug like levothyroxine, ask your pharmacist to fill your prescription with the same generic brand each time. Don’t switch unless you have to.
- Use price tools. Apps like GoodRx compare prices across pharmacies. Sometimes the brand is cheaper than the generic at one store. Always check.
- Speak up if something feels off. If you notice new side effects after switching to a generic-especially for thyroid, seizure, or blood thinner meds-tell your doctor. It’s not all in your head.
Who’s Making These Drugs?
Most generic drugs sold in the U.S. are made overseas. About 80% of the active ingredients come from countries like India and China. The FDA inspects these factories, but a 2023 government report found that 18% of foreign facilities had at least one quality issue during inspection-compared to 8% of U.S. ones.
That doesn’t mean they’re unsafe. Most are fine. But it does mean quality control isn’t perfect. That’s why drug shortages happen. In 2022, there were 178 active shortages of generic drugs, mostly because of production problems or supply chain issues.
Major generic makers include Teva, Sandoz, and Viatris. They’re not shady companies. They’re big, regulated businesses. But their business model relies on volume and low prices. That’s why they can afford to sell a month’s supply of generic statins for $1.
What’s Next?
The FDA is working to speed up approval of generic drugs-from 14 months down to 10. They’re also launching new programs to handle complex generics, like inhalers and creams, which are harder to copy than pills.
By 2030, the World Health Organization expects 70% of all prescriptions worldwide to be for generics. That’s up from 56% today. Why? Aging populations. Rising drug prices. And the fact that generics just work.
The real question isn’t whether generics are safe. It’s whether we’re using them wisely. For most of us, they’re the smart, safe, and affordable choice. For a small group, consistency matters more than cost.
Know your meds. Know your body. And don’t be afraid to ask questions.
Are generic drugs as effective as brand-name drugs?
Yes, for the vast majority of medications. The FDA requires generics to have the same active ingredient, strength, dosage form, and bioequivalence as the brand-name version. Studies show they work the same way in the body. For common drugs like blood pressure pills, diabetes meds, and antibiotics, patients report nearly identical results.
Why do generic pills look different from brand-name ones?
By law, generic manufacturers can’t copy the exact appearance of brand-name drugs to avoid trademark issues. That means different colors, shapes, or markings. But the active ingredient is the same. Always check the label for the drug’s name and dosage, not the pill’s appearance.
Can switching to a generic cause side effects?
For most people, no. But for certain drugs with a narrow therapeutic index-like levothyroxine, warfarin, or some seizure medications-switching between different generic brands can lead to changes in how the drug is absorbed. This may cause side effects or reduced effectiveness. If you notice new symptoms after switching, talk to your doctor.
Are generic drugs made in the U.S.?
Some are, but most active ingredients come from overseas-primarily India and China. The FDA inspects all manufacturing facilities, domestic and foreign, and requires them to meet the same quality standards. However, inspections have found slightly more issues at foreign facilities. That doesn’t mean they’re unsafe, but it’s why drug shortages sometimes happen.
How can I save money on my prescriptions?
Always ask if a generic version is available. Use price-comparison tools like GoodRx, which can show you the lowest price at nearby pharmacies. Sometimes the brand-name drug is cheaper than the generic at a specific store. Also, ask your doctor if you can switch to a lower-cost generic alternative, especially for long-term medications.
What are biosimilars, and are they the same as generics?
Biosimilars are the generic-like versions of biologic drugs-complex medicines made from living cells, like Humira or Enbrel. Unlike traditional generics, they’re not exact copies but are highly similar and equally safe and effective. They’re typically 15%-35% cheaper than the brand. While they’re growing fast, they still make up only a small portion of prescriptions.
Can I ask my pharmacist to always fill my prescription with the same generic brand?
Yes. You have the right to request the same generic manufacturer each time, especially for drugs with a narrow therapeutic index. Pharmacists can often honor this request, though it may require a note from your doctor. Consistency matters more than cost for certain medications.
Why do some doctors still prescribe brand-name drugs?
Sometimes it’s habit. Other times, they’re following a patient’s history-if a generic switch caused issues in the past. Or they’re prescribing a drug where no generic exists yet. In some cases, insurance or pharmacy benefit managers push brand-name drugs for rebates. But most doctors will switch you to a generic if you ask and it’s appropriate.





Comments
Chris Wallace
December 3, 2025 AT 18:23Man, I’ve been on generic levothyroxine for years and never had an issue. But my aunt switched brands last year and started having heart palpitations-she thought it was stress until her endo pointed out the switch. Now she sticks to one brand, even if it costs a few bucks more. I get why people want to save money, but when it’s your thyroid, consistency beats savings every time.
Also, I used to think generics were just cheap knockoffs until I read that FDA study showing the absorption difference is usually under 4%. That’s wild. It’s like buying a used car that’s mechanically identical to a new one, just without the shiny badges.
And yeah, the pill looks different? Totally normal. I keep a little note in my phone with the color and shape of each med. Saved me once when I thought my new bottle was a different drug entirely.
Bottom line: generics are fine for most stuff. But if you’re on something with a narrow window, don’t gamble. Ask for the same maker. Your body will thank you.
william tao
December 3, 2025 AT 21:07One must question the regulatory integrity of the FDA’s bioequivalence standards, particularly in light of the documented inconsistencies in manufacturing protocols across international facilities. The data, while statistically significant, fails to account for individual pharmacokinetic variance-a factor that, in clinical practice, is not negligible. One cannot reduce human physiology to a mean absorption curve.
Sandi Allen
December 5, 2025 AT 14:36Wait-so you’re telling me the government says it’s ‘safe’ because the numbers look good on paper?? But the factories are in India and China?? And they get inspected… sometimes?? And 18% have issues?? And you think that’s okay??
WHAT IF THEY’RE USING TOXIC FILLERS?? WHAT IF THE ACTIVE INGREDIENT IS CUT?? WHAT IF THEY’RE JUST SHIPPING PLACEBO PILLS??
I’VE SEEN THE DOCUMENTARIES. I’VE READ THE WHISTLEBLOWER REPORTS. THIS ISN’T ‘SAFETY.’ THIS IS CORPORATE GREED WITH A FDA STAMP ON IT.
And don’t even get me started on ‘biosimilars’-that’s just Big Pharma’s way of keeping you hooked while pretending to save you money. They’re not ‘similar’-they’re engineered to be slightly off so you keep buying the brand. It’s a scam. I only take brand-name. Even if I have to sell a kidney.
John Webber
December 6, 2025 AT 11:45generic drugs are fine but sometimes u get a bad batch and u feel weird. like last month i switched to a new generic for my blood pressure and i felt dizzy all day. called my doc, he said switch back. so i did. no big deal. but why do they make the pills look so different? i keep thinking i’m taking something else. just write the name on the pill like the brand does. simple.
also why do pharmacies switch brands without telling u? that’s just rude. i’m not a robot. i notice stuff.
Shubham Pandey
December 7, 2025 AT 02:18Generic works. Save money. No drama.
Elizabeth Farrell
December 7, 2025 AT 04:15I just want to say how important it is that people feel empowered to ask questions about their meds. So many of us are told, ‘Just take it,’ and then we’re too afraid to speak up when something feels off.
My mom was on warfarin for years. When the pharmacy switched her generic brand, she started having bruising all over her arms. She didn’t say anything for weeks because she didn’t want to ‘bother’ anyone. Finally, she wrote down her symptoms and took them to her doctor. Turns out, the new version was absorbed a bit faster-and her INR spiked.
She’s been on the same generic since. The pharmacist even keeps a sticky note in her file now. It’s not about being picky. It’s about being your own advocate.
If you’re on a narrow therapeutic index drug, please, please ask for consistency. And if your pharmacist says ‘no,’ ask for a note from your doctor. You’re not being difficult-you’re being smart.
Sheryl Lynn
December 8, 2025 AT 10:33How quaint. We’ve reduced the sacred science of pharmacology to a Walmart aisle negotiation. ‘Oh, I’ll just grab the generic-it’s 85% cheaper!’ as if the molecular architecture of human physiology were a discount coupon.
One must appreciate the exquisite artistry of the original formulation-the proprietary excipients, the slow-release matrices, the crystalline polymorphs painstakingly engineered to optimize bioavailability. These are not mere ‘chemical copies.’ They are symphonies. And generics? They’re the karaoke version.
And yet… I confess, I do use them. For antibiotics. For ibuprofen. For the mundane. But for my mood stabilizer? I pay the premium. Because some things are not meant to be commodified. Some things deserve the original score.
Paul Santos
December 9, 2025 AT 23:11Interesting how we fetishize ‘bioequivalence’ while ignoring the ontological gap between a molecule and its phenomenological experience in vivo. The FDA’s 25% variance allowance is less a scientific threshold than a neoliberal concession to capital.
Also, the pill color thing? Classic semiotic dissonance. We’ve conditioned patients to equate visual identity with therapeutic authenticity. Hence the 65% error rate. We’re not just prescribing drugs-we’re prescribing visual rituals. 🤔
And biosimilars? The new frontier of pharmacological mimicry. Aesthetic approximation without ontological fidelity. The pharmaceutical equivalent of AI-generated art. Beautiful. But not alive. 🧬
Eddy Kimani
December 11, 2025 AT 21:08Just read the 2016 JAMA study again-average absorption difference was 3.5% with a 95% CI of ±2.1%. That’s tighter than most drug-drug interactions we worry about clinically. And for NTI drugs, the real issue isn’t the generic itself-it’s the lack of standardized labeling across manufacturers. The FDA needs to mandate a unique identifier on the pill, like a QR code that links to the batch and excipient list.
Also, most people don’t realize that 40% of brand-name drugs are made in the same factories as generics. It’s the same line, same QC, same inspectors. The only difference is the label. So when someone says ‘brand is better,’ they’re often just paying for marketing, not medicine.
John Morrow
December 12, 2025 AT 16:58Let’s be brutally honest: the entire generic drug system is a controlled failure. The FDA’s bioequivalence standards are archaic. They measure AUC and Cmax, but ignore gut microbiome variability, epigenetic expression, and hepatic enzyme polymorphisms-factors that can alter drug response by 300% in certain populations.
And yet, we’re told to trust a 3.5% average? That’s like saying a car is ‘equivalent’ because both a Tesla and a Yugo hit 60 mph in 12 seconds. One is engineered for precision. The other is engineered for cost.
Also, 80% of API sourced from China? In a world where supply chains are weaponized? This isn’t healthcare. It’s geopolitical roulette. And you’re the pawn.
Kristen Yates
December 12, 2025 AT 17:34I’m from a country where people don’t have the luxury of choosing between brand and generic. We take what’s available. And in my community, generics are lifesavers. My neighbor’s son with epilepsy got seizure control after switching to a consistent generic brand-after months of hospital visits because the pharmacy kept changing it.
It’s not about being elite or paranoid. It’s about access. I’m grateful for generics. I just wish we made it easier for people to get the same one every time. No one should have to fight for consistency just to stay alive.
Saurabh Tiwari
December 13, 2025 AT 14:35Generic = good 💯
Save money, same medicine, no need to overthink
But if u feel weird after switch, talk to doc. simple 😊
Chris Wallace
December 15, 2025 AT 11:29Yeah, I saw your comment about the pill color confusion. I actually had a friend who stopped taking her blood pressure med because the generic looked like her old anxiety pill. She didn’t realize they were different until she passed out at work.
Now she keeps a photo of each pill on her phone. And she texts her pharmacist every time she gets a refill: ‘Same brand as last time?’
It’s not paranoia. It’s just… smart. Especially when you’re on multiple meds. I think pharmacies should print the manufacturer name right on the bottle. Not just the pill shape. The actual company. Like ‘Made by Teva’ or ‘Sandoz.’
It’s a tiny thing. But it could prevent a lot of panic.